How to draw bohr diagrams
Table of Contents
Table of Contents
Have you ever struggled with creating a Bohr diagram? It can be overwhelming, especially if you’re new to chemistry. But don’t worry - drawing a Bohr diagram is easier than you think. In this article, we’ll guide you through the process, step by step.
The Pain Points of Creating a Bohr Diagram
Creating a Bohr diagram can be a difficult task, particularly if you’re trying to visualize something that you can’t see. The process starts with identifying how many electrons are in a particular atom. After that, it’s about figuring out which energy levels those electrons are located on and how many electrons are on each level.
How to Draw a Bohr Diagram
The first step in drawing a Bohr diagram is to determine the number of protons and electrons in the atom. The bottom circle of the Bohr diagram represents the first energy level and should contain no more than two electrons. The second energy level can hold up to eight electrons and is represented by the second circle. Continue adding circles for each energy level, with each level farther from the nucleus having more energy than the previous level.
Summary of Main Points
A Bohr diagram shows how many electrons are in each energy level of an atom. To draw a Bohr diagram, identify the number of electrons in the atom and place them in the corresponding energy level circles.
Example with Personal Experience
When I was first learning how to draw Bohr diagrams, I struggled to understand the concept of energy levels. However, once I realized that each level farther from the nucleus has more energy than the last, it was easier to understand how the electrons were arranged within the atom.
Further Explanation of Bohr Diagrams
Bohr diagrams demonstrate the arrangement of electrons within an atom. It’s important to keep in mind that electrons always fill up the lowest energy levels first before moving on to higher ones. Additionally, each energy level can only hold a certain number of electrons before they move to a higher energy level.
Electron Configuration
The electron configuration of an atom represents the number of electrons in each energy level. It’s written out using a series of numbers and letters, with the numbers representing the energy levels and the letters representing the number of electrons in each energy level.
Tips for Drawing a Bohr Diagram
One helpful tip for drawing a Bohr diagram is to start with the innermost circle and work your way outwards, adding electrons into each circle as you go. Additionally, remember to place no more than two electrons in the first energy level and a maximum of eight electrons in the second energy level.
Question and Answer
Q: Why are Bohr diagrams important?
A: Bohr diagrams show the arrangement of electrons within an atom, allowing scientists and students to better understand how different atoms behave.
Q: What is the maximum number of electrons that can fit in the third energy level of a Bohr diagram?
A: The maximum number of electrons that can fit in the third energy level is 18.
Q: How do you know how many energy levels to include in a Bohr diagram?
A: The number of energy levels in a Bohr diagram corresponds to the number of electron shells in the atom.
Q: What is the difference between the Bohr model and the Lewis structure?
A: The Bohr model shows the arrangement of electrons within an atom, while the Lewis structure shows how electrons are shared between atoms in a molecule.
Conclusion of How to Draw a Bohr Diagram
Creating a Bohr diagram may seem daunting at first, but with the right approach, anyone can do it. Remember to identify the number of electrons in the atom, place them in the corresponding energy levels, and keep in mind that each level can only hold a certain number of electrons before they move to a higher energy level. Happy diagramming!
Gallery
How To Draw Bohr Diagrams - Presentation Chemistry
Photo Credit by: bing.com /
How To Draw Bohr Diagrams - Presentation Chemistry
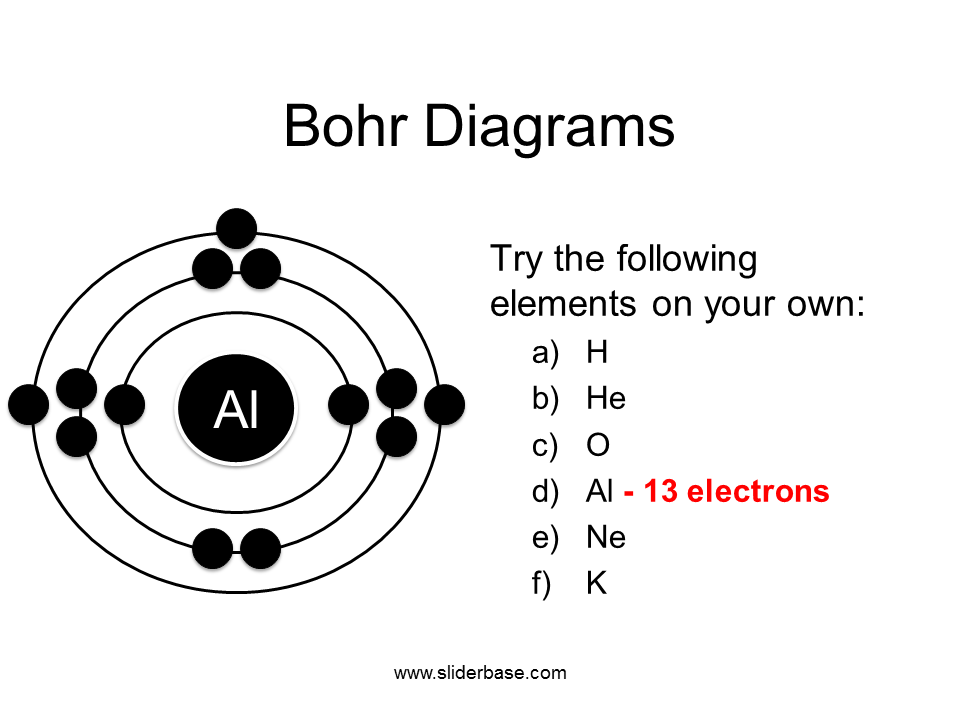
Photo Credit by: bing.com / bohr diagrams diagram dot argon draw elements he lewis electron electrons al try following ne own presentation sliderbase
How To Draw Bohr Diagrams – A Step By Step Tutorial – Middle School

Photo Credit by: bing.com / bohr
Bohr Model Drawing Of Oxygen At PaintingValley.com | Explore Collection
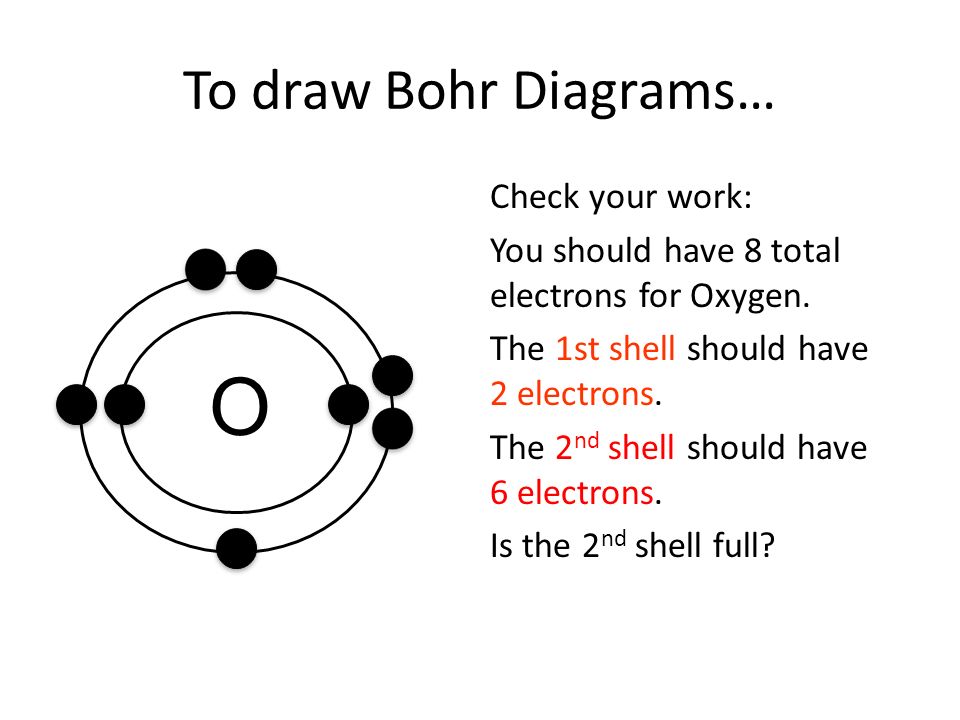
Photo Credit by: bing.com / bohr model oxygen drawing draw paintingvalley elements diagrams atoms
How To Draw Bohr Diagrams (slideshare)

Photo Credit by: bing.com / bohr carbon electrons nucleus