How to draw lewis dot structure
Table of Contents
Table of Contents
If you’re a student of chemistry, no doubt you’ve encountered lewis dot diagrams. They can seem intimidating at first, but with a bit of practice, they become much easier to draw. In this article, we’ll dive deep into how to draw lewis dot diagrams and related keywords, so you can ace your next chemistry exam.
Pain Points surrounding How to Draw Lewis Dot Diagrams
If you’re struggling with lewis dot diagrams, you’re not alone! They can be difficult to wrap your head around if you’re not used to drawing them. Common issues include understanding how to find the valence electrons and determining the correct placement of electrons around the atoms. But fear not - we’ll tackle these pain points head-on in this article.
Answering the target of How to Draw Lewis Dot Diagrams
At its core, a lewis dot diagram is a visual representation of an atom’s valence electrons. These are the electrons in the outermost energy level of the atom and are responsible for how the atom reacts chemically. To draw a lewis dot diagram, you’ll need to determine how many valence electrons an atom has and then place dots around the atom’s symbol to represent those electrons.
Summary of How to Draw Lewis Dot Diagrams and Related Keywords
To draw a lewis dot diagram, you’ll need to remember the following steps:
- Determine how many valence electrons the atom has
- Place one dot on each side of the atom’s symbol
- Place any additional dots around the sides of the atom’s symbol, following a clockwise direction
- If there are more than four valence electrons, pair the electrons up until each side has a maximum of two dots
With these steps in mind, let’s dive deeper into how to draw lewis dot diagrams and related keywords.
Understanding Valence Electrons
Before we can draw a lewis dot diagram, we must first understand valence electrons. These electrons are important because they are the outermost electrons in an atom and are responsible for how the atom reacts chemically with other elements. The number of valence electrons an atom has will determine the placement and number of dots on the diagram.
For example, let’s consider the element fluorine. Flourine has 7 electrons in total, but only one electron in its outermost shell. This single electron represents the valence electron, and will be denoted in the lewis dot diagram with a single dot on one side of the fluorine symbol.
 Using Lewis Dot Diagrams to Determine Bonding
Using Lewis Dot Diagrams to Determine Bonding
Lewis dot diagrams are useful not only for determining the number and placement of valence electrons, but for predicting how atoms will bond with each other. A covalent bond, for example, is formed when two atoms share electrons. This sharing can be represented visually in the lewis dot diagram by overlapping dots between the two atoms. Alternatively, if two elements have a complete transfer of electrons, they will form an ionic bond.
Let’s consider the example of water. The lewis dot diagram for water will show two hydrogen atoms bonded to one oxygen atom. The hydrogen has one valence electron and the oxygen has six valence electrons. The diagram will show the sharing of electrons between the atoms and help us understand the formation of the molecule.
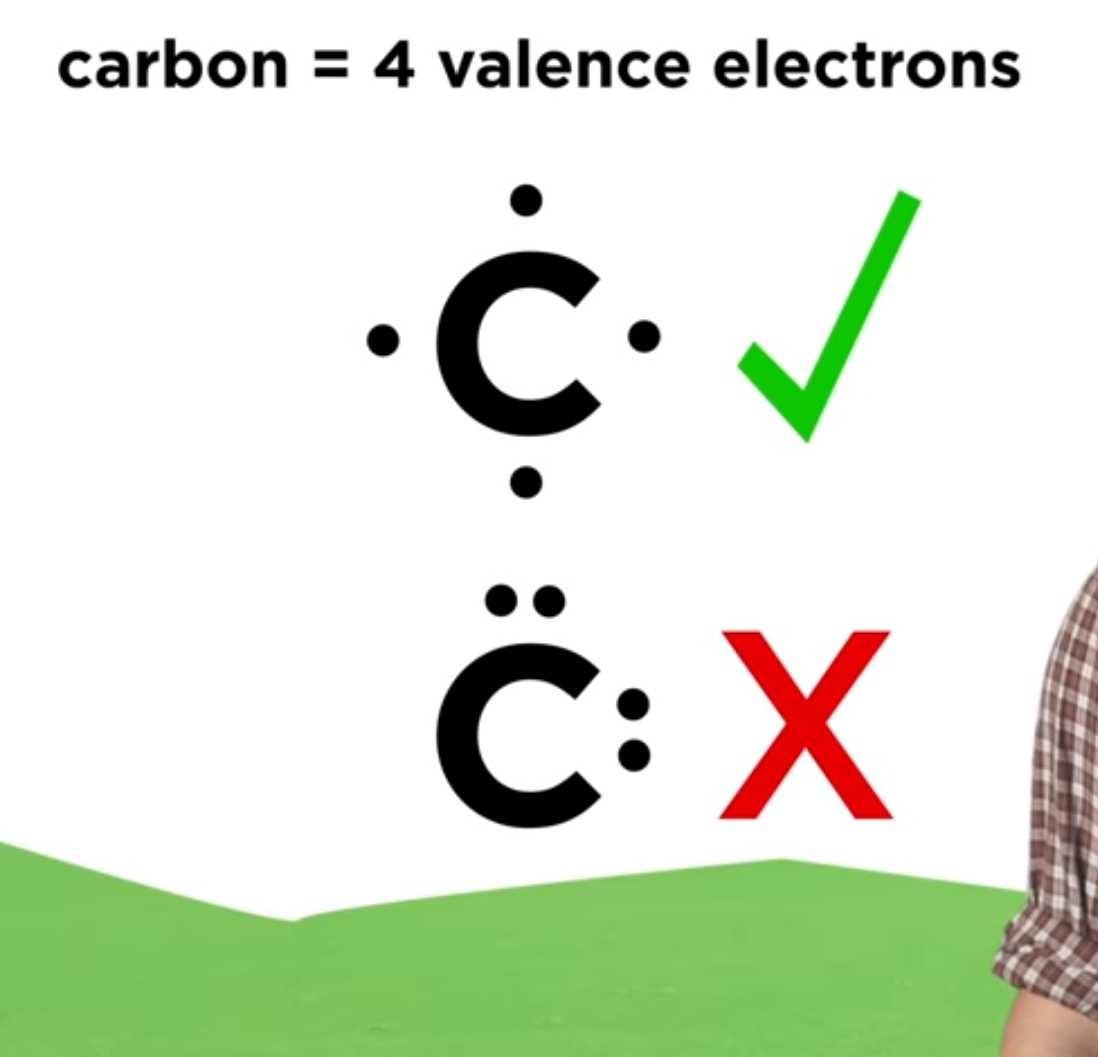 Common Errors to Avoid when Drawing Lewis Dot Diagrams
Common Errors to Avoid when Drawing Lewis Dot Diagrams
One of the most common errors when drawing lewis dot diagrams is miscounting the number of valence electrons an atom has. Remember, the number of valence electrons an atom has is determined by its group number on the periodic table. Another common error is failing to follow the clockwise pattern when placing additional dots on the diagram.
It’s also important to note that when forming covalent bonds, you should remember that both atoms in the bond should have the correct number of valence electrons in their lewis dot diagrams. This will ensure a stable molecule is formed.
Question and Answer
1. How do I determine the number of valence electrons an atom has?
The number of valence electrons an atom has is determined by its group number on the periodic table.
2. Can an atom have more than 8 valence electrons in its lewis dot diagram?
Yes, atoms in the third row of the periodic table and beyond have d subshells, which allow for more than eight valence electrons in the lewis dot diagram.
3. Can lewis dot diagrams predict the shape of a molecule?
Yes, the shape of a molecule can be predicted by the lewis dot diagram and the placement of the atoms and lone pairs of electrons on the molecule.
4. How do I know if an atom will form a covalent or ionic bond?
The electronegativity difference between two atoms can determine if they will form a covalent or ionic bond. If the electronegativity difference is greater than 1.7, the atoms will form an ionic bond. Otherwise, they will form a covalent bond.
Conclusion of How to Draw Lewis Dot Diagrams
Drawing lewis dot diagrams may seem intimidating, but with practice, anyone can master the technique. Remember to always count the correct number of valence electrons and to follow the clockwise pattern when placing additional dots on the diagram. By mastering the basics of lewis dot diagrams, you’ll be well on your way to understanding the fundamentals of chemistry.
Gallery
Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures - YouTube

Photo Credit by: bing.com / lewis dot draw structures diagrams easy made
3 Ways To Draw Lewis Dot Structures - WikiHow

Photo Credit by: bing.com / wikihow electrons
ELECTRON DOT DIAGRAM - Unmasa Dalha

Photo Credit by: bing.com / dot electron diagram lewis diagrams chemistry bohr interrupt need rutherford
How To Draw Lewis Dot Structure

Photo Credit by: bing.com / lewis structure cs2 dot carbon draw electrons diagram valence drawing oxygen steps every square side each look
Patterns In Electron Configuration Worksheet Answer Key - Lewis Dot

Photo Credit by: bing.com / diagrams configuration rubidium configurations